Our Product
- Overview
- Process
- Application
- Spesification
Overview
Phenol solution or benzenol comprises of a white crystalline mass dissolved in an aqueous solution that comes with the chemical formula of C6H5OH. The solution may be colorless to slightly pink in color with a distinctive phenol odor and a sharp burning taste. Aqueous solutions of phenol are weakly acidic and turn blue litmus slightly to red. Phenol (C6H6O or C6H5OH) is a colorless to light-pink, crystalline solid with a sweet, acrid odor. Exposure to phenol may cause irritation to the skin, eyes, nose, throat, and nervous system. Some symptoms of exposure to phenol are weight loss, weakness, exhaustion, muscle aches, and pain.
Uses
The major uses of phenol, consuming two thirds of its production, involve its conversion to precursors for plastics. Condensation with acetone gives bisphenol-A, a key precursor to polycarbonates and epoxide resins. Condensation of phenol, alkylphenols, or diphenols with formaldehyde gives phenolic resins, a famous example of which is Bakelite. Partial hydrogenation of phenol gives cyclohexanone, a precursor to nylon. Nonionic detergents are produced by alkylation of phenol to give the alkylphenols, e.g., nonylphenol, which are then subjected to ethoxylation. Phenol is also a versatile precursor to a large collection of drugs, most notably aspirin but also many herbicides and pharmaceutical drugs. Phenol is a component in liquid–liquid phenol–chloroform extraction technique used in molecular biology for obtaining nucleic acids from tissues or cell culture samples. Depending on the pH of the solution either DNA or RNA can be extracted.
- Medical
Phenol was widely used as an antiseptic. Its use was pioneered by Joseph Lister. From the early 1900s to the 1970s it was used in the production of carbolic soap. Concentrated phenol liquids are used for permanent treatment of ingrown toe and finger nails, a procedure known as a chemical matrixectomy. The procedure was first described by Otto Boll in 1945. Since that time phenol has become the chemical of choice for chemical matrixectomies performed by podiatrists. Concentrated liquid phenol can be used topically as a local anesthetic for otology procedures, such as myringotomy and tympanotomy tube placement, as an alternative to general anesthesia or other local anesthetics. It also has hemostatic and antiseptic qualities that make it ideal for this use. Phenol spray, usually at 1.4% phenol as an active ingredient, is used medically to treat sore throat. It is the active ingredient in some oral analgesics such as Chloraseptic spray, TCP and Carmex. - Niche uses
Phenol is so inexpensive that it also attracts many small-scale uses. It is a component of industrial paint strippers used in the aviation industry for the removal of epoxy, polyurethane and other chemically resistant coatings. Due to safety concerns, phenol is banned from use in cosmetic products in the European Union and Canada.
Manufacturing Proses
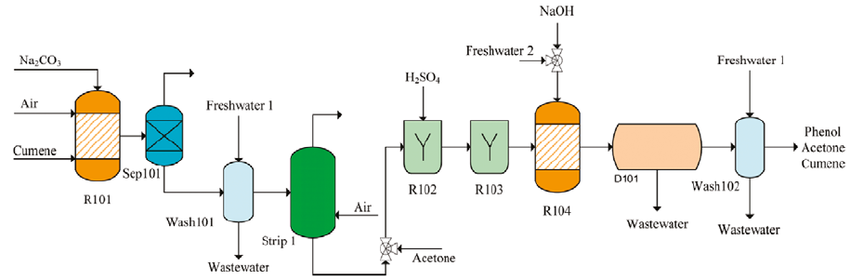
The vast majority of phenol is made by the cumene process, which comprises of three stages:
1) Production of Cumene
Cumene is produced via the Friedel-Crafts reaction by reacting benzene and propene with an acid catalyst such as ZSM-5, under pressure (ca 10 atm) in a fixed bed reactor.
2) Conversion of Cumene to Cumene hydroperoxide
Cumene is then oxidized with air to give the hydroperoxide and the reaction is autocatalyzed by cumene hydroperoxide. The reaction takes place at temperatures between 350-390 K and 1-7 atm pressure, the latter to retain the system in the liquid phase. At higher temperatures, the hydroperoxide is unstable and could decompose violently.
3) Decomposition of Cumene hydroperoxide
Finally, the hydroperoxide is mixed with sulfuric acid at 313-373 K to give phenol and propanone. The products are separated by distillation and a product yield of 85-87% is obtained, based on benzene.
Applications
Phenol is used to make chemical intermediates for a wide range of other applications, such as:
- Dyes
Phenol is used to produce phenylamine (aniline) which is used in the manufacturing of dyes. - Pharmaceutical Industry
Phenol is used in the production of antiseptics such as 2,4-and 2,6-dichlorophenols. - Plastics
Phenol is used to manufacture bisphenol A, which is used to make polycarbonates for plastics. - Synthetic Polymers
Phenol is used to produce epoxy resins for paints, coatings and mouldings.
Spesifications
PRODUCT IDENTIFICATION
- Chemical Name
- Chemical Formula
- CAS No.
- HS Code
- Synonyms
- Molecular Weight
- Phenol
- C6H5OH
- 108-95-2
- 2907.00.00
- Monohydroxybenzene, Benzenol, Phenyl hyroxide, Carbolic Acid
- 94.11 g mol -1
PHYSICAL & CHEMICAL PROPERTIES
- Appearance
- Odor
- Boiling Point
- Melting Point
- Critical Temperature
- Vapor Density
- Colorless to light pink liquid
- Sharp, sweet, tangy, medicinal
- 182°C (359.6°F)
- 42°C (107.6°F)
- 694.2°C (1281.6°F)
- 3.24 (Air=1)
